INEOS modular membrane electrolysers are at the forefront of innovation, offering operators a highly efficient and sustainable method of electrolysis. Featuring an ultra-low power comsumption of 1962 kWH/te of NaOH at 6kA/m2*, and a class leading output of 52,000 MTPA NaOH per electrolyser. Learn how INEOS electrolysers are constructed and operate below.
* Expected value @ 385mbarg, 90°C and 32wt% NaOH, subject to coating selection
**Based on 350 days operation and 7kA/m2
The Modular Membrane Electrolyser Process
Membrane cell electrolysers feature a sealed module that consist of two chambers, separated by a flexible cation exchange membrane, to prevent chlorine and hydrogen gasses from mixing. The two chambers are formed by the anode and cathode pan assemblies respectively, and once connected to an electricity supply, is where the electrolysis takes place.
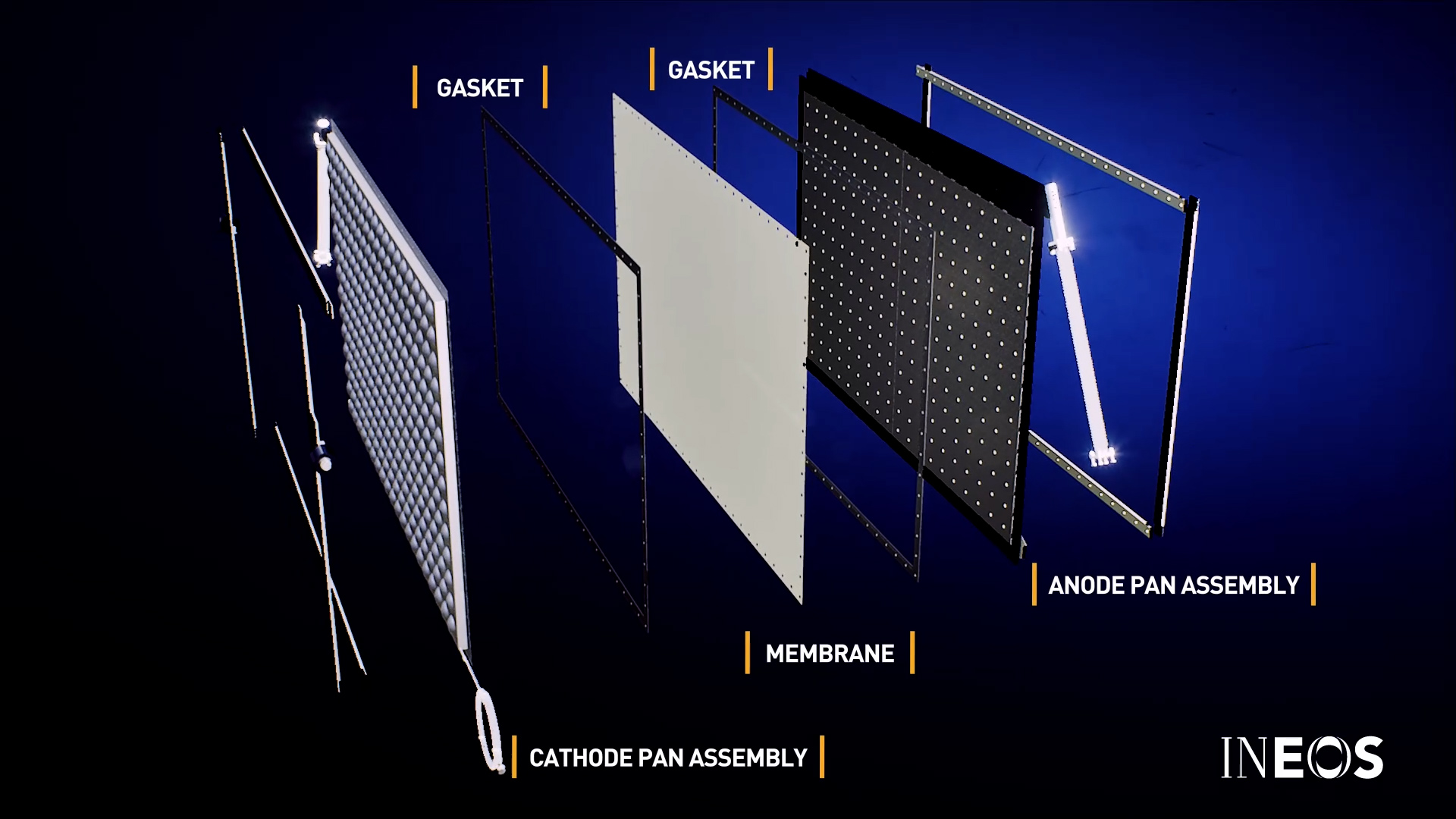
The anode and cathode assemblies (electrodes) are critical design features of an electrolyser, and must be manufactured from special materials to prevent corrosion by the chlorine.
The anode is where the chlorine is generated and was historically manufactured from graphite or platinum alloys. Modern electrolysers feature anodes made from titanium for corrosion resistance. The cathode element is typically less sensitive to corrosion, and is often manufactured from nickel, although stainless steel can be used.
Additionally, each electrode is coated to improve power performance and efficiency of the electrolysis process.
- Cathode pan coatings electro-catalytically promote H2 evolution and provide resistance to impurity poisoning and reverse currents at shut-down.
- Anode pan coatings are for the electrocatalytic promotion of Cl2 evolution and have superior alkali wear performance and lower exit brine chlorate values
The flexible membrane in the centre of each module is fully supported in a ‘zero-gap’ design to prevent flapping or pinching of the membrane material (cross-section of a module assembly detailed below).
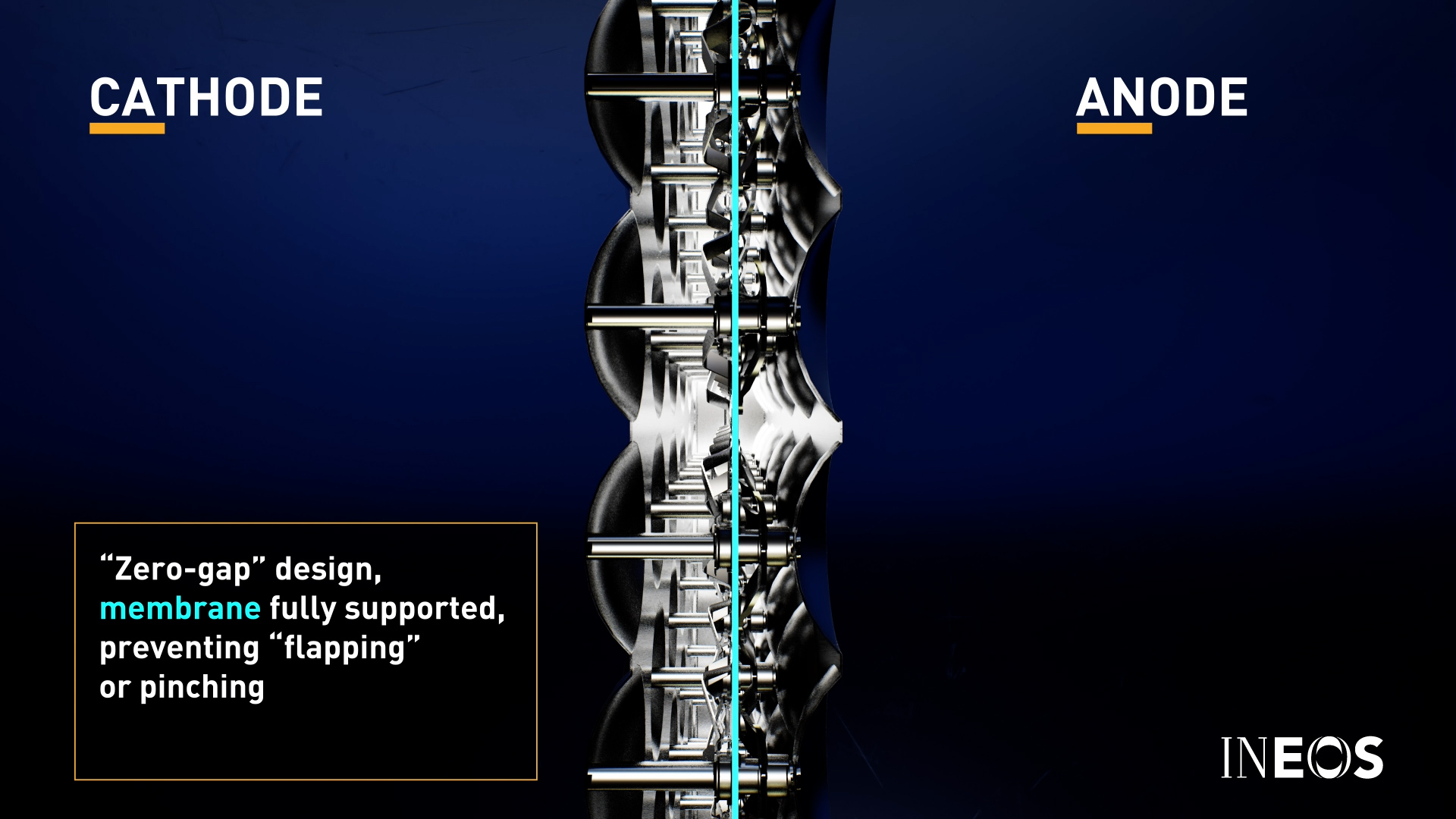
An industrial-scale modular electrolyser, such as the INEOS BICHLOR Electrolyser, consists of such modules arranged electrically in series within a frame. Typically a number of electrolysers are installed in a cell room side by side, with the quantity designed to meet the overall desired capacity of the plant.

Operation
- Electrolyte (brine to the anodes and caustic to the cathodes) enters through feed tubes, full wetting the membrane in the centre of the cell, for inherently safer operation :

- Electrical current begins to flow through the metal spiders and electrodes of the electrolysis cell

- Sodium (Na+) or Potassium (K+) ions pass through the semi-permeable ion exchange membrane
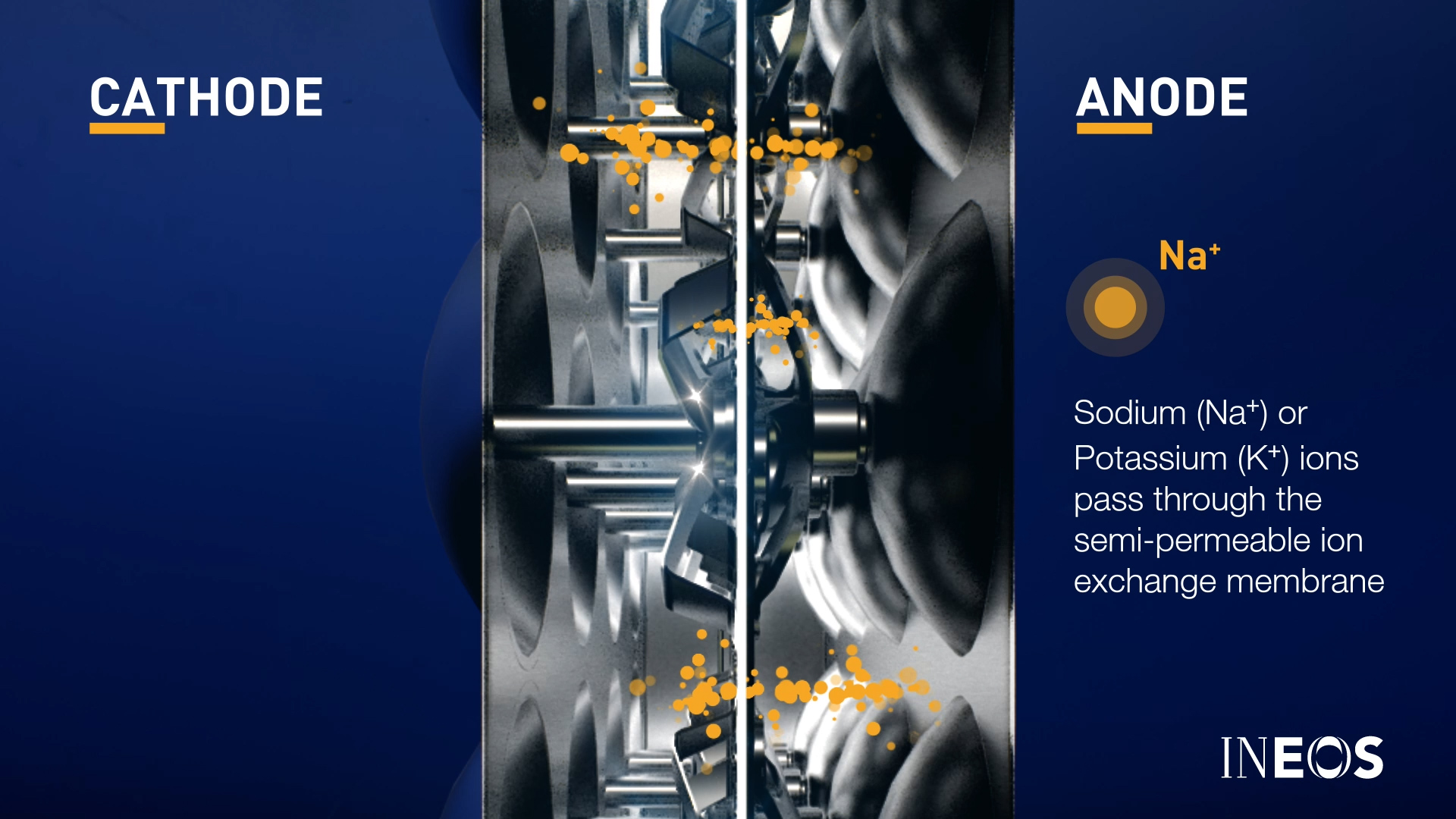
- Chlorine gas bubbles form at the anode and rise to the top of the chamber.
Hydrogen gas bubbles form at the cathode and rise to the top

- Chlorine and hydrogen gasses are then extracted from the top of their respective chambers
The overall reaction for the electrolysis of brine is : 2NaCl + 2H2O → Cl2 + H2 + 2NaOH